The SMART IRB platform has replaced the JDP Coversheet for documenting review-reliance for graduate student research proposed for thesis or dissertation that engages both SDSU and UCSD in human Subjects research. See the Guidance page for more details.
What's New!
January 12, 2026
Guidance on the Use of AI in Human Subjects Research
A new guidance document on the Use of Artificial Intelligence (AI) in Human Subjects Research is now available. This guidance outlines important requirements and considerations for researchers who use AI tools at any stage of study design, data analysis, or document preparation.
The document addresses key areas including:
- Data privacy and security, including restrictions on the types of data that may be entered into AI platforms and the requirement to use SDSU-licensed AI tools.
- Algorithmic bias, with expectations for human review and validation of AI outputs as part of the IRB application.
- Informed consent, including investigator responsibilities when AI is used to draft consent materials and the requirement to disclose AI use to research participants.
Researchers planning to use AI or machine learning in human subjects research are strongly encouraged to review this guidance and ensure their protocols and consent documents are compliant prior to submission.
Effective February, iRIS has been updated with a new application version to capture the scope and nature of AI/ML use in research.
When you encounter the new section, you will be asked to provide details if you select "Yes" to using AI/ML tools.
Requirements include:
- Purpose of Use: Explain why AI is being used and the specific tasks it will perform (e.g., data analysis, recruitment, or drafting materials).
- Data Specifics: Specify the type of data involved (identifiable, coded, or de-identified).
Tools & Platforms: List all specific tools, algorithms, and platforms (e.g., SDSU-licensed versions of ChatGPT, CoPilot, or Gemini). - Bias & Fairness: Describe steps taken to minimize algorithmic bias and ensure equitable results.
- Human Oversight: Detail how the PI or research staff will review and validate AI-generated outputs.
April 15, 2025
Human Research Recruitment Materials Policy
This document outlines the official policy of the SDSU Institutional Review Board (IRB) regarding the creation and use of recruitment materials in human subjects research. Effective as of April 15, 2025, the policy provides clear guidance to SDSU Principal Investigators on the required content, presentation standards, and review procedures for recruitment advertisements such as flyers, social media posts, and other public communications. The policy aims to ensure that recruitment materials accurately and ethically inform prospective research participants, align with the approved research protocol, and protect the rights and interests of human subjects.
April 4, 2025
We are pleased to share a crucial resource for human subjects researchers at SDSU: “To Include or Not Include: The Injury Paragraph.” This document clarifies when and why the injury paragraph must be included in informed consent forms.
According to SDSU IRB guidelines, the injury paragraph is required only for studies deemed Greater than Minimal Risk, as well as specific categories under Exempt (6) and Expedited reviews (1–4). This ensures participants are fully informed about what happens in the unlikely event of injury during the study.
Please review this document thoroughly and refer to it when drafting your study consent
forms.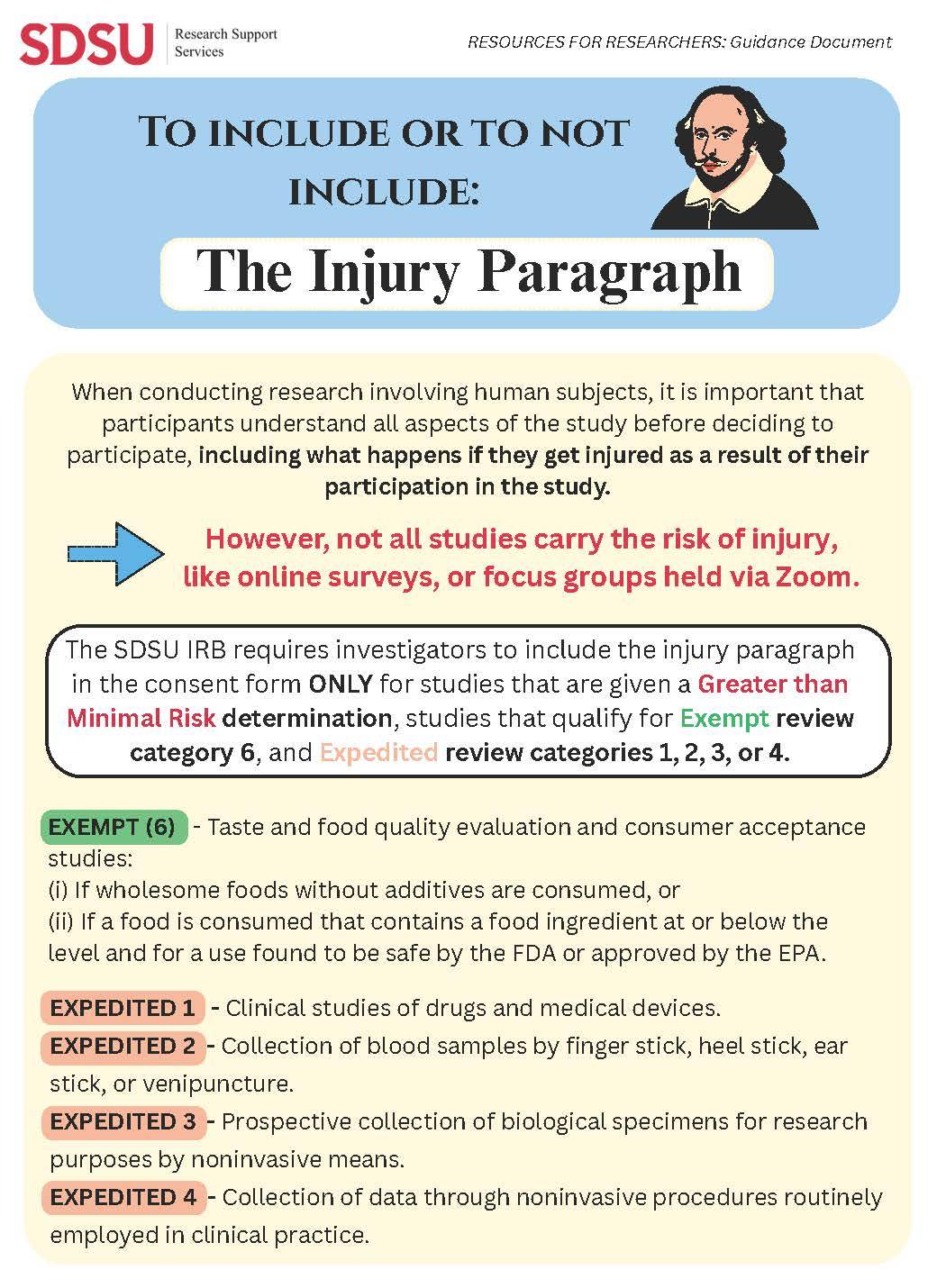
March 21, 2023
The SDSU HRPP Office has created a new IRB-approved Consent Translation Policy, effective January 2024, for use in human participant research protocols.
Translation of Informed Consent Documents Policy
- Ensures informed consent be understood by research participants
- Requires translated documents reflect language of study population
- Provides options for translation services
- Defines translation terminology
February 15, 2023
Revised consent templates are now available on the SDSU IRB SharePoint site.
October 19, 2022
Supplemental Information to the NIH Policy for Data Management and Sharing: Protecting Privacy When Sharing Human Research Participant Data
To advance efforts under its new Data Management and Sharing Policy (DMS Policy), NIH is providing supplemental information assisting researchers in addressing privacy considerations when sharing human research participant data. This information is not intended to provide a guide for compliance with regulatory requirements nor is it establishing binding rules for NIH awardees, but instead provides a set of principles, best practices, and points to consider for creating a robust framework for protecting the privacy of research participants when sharing data.