IRB Review-Reliance Process
Overview of the Process
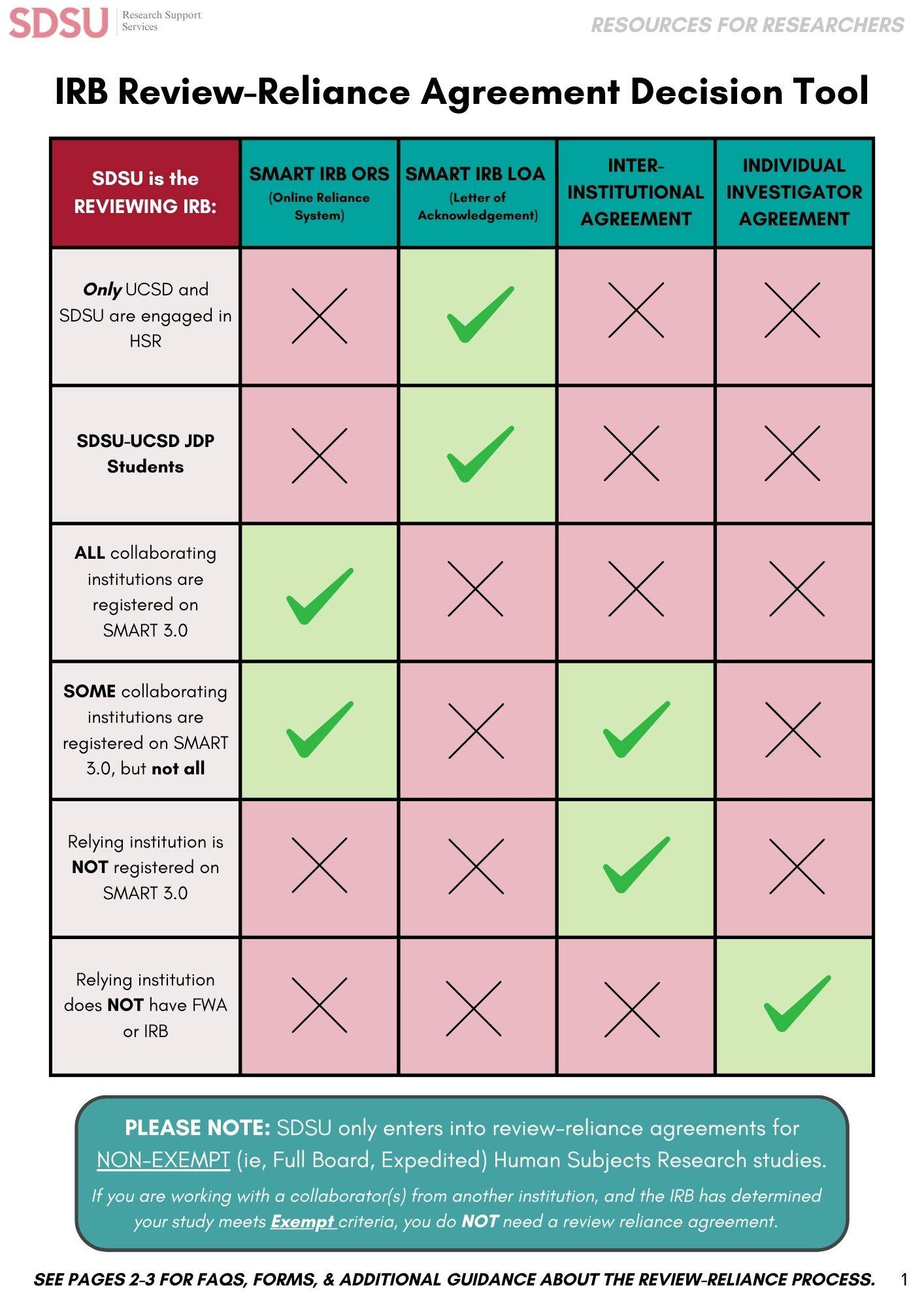
The process will differ depending on each stakeholder's role in the process. Watch the video of the SMART IRB Review-Reliance process below for more information. Investigators can sign up to gain access for cooperative, multi-site studies to be reviewed under the agreement where SDSU is the reviewing IRB or is relying on another participating IRB for review.
Process when the SDSU IRB Relies on Another IRB -- SDSU is not the Reviewing IRB.
*In instances where the SDSU IRB relies on another IRB for review, the process will differ depending on the reviewing institution's policies and processes. SDSU investigators should work with their collaborators at the proposed reviewing institution to determine the process and their responsibilities when the SDSU IRB relies on another IRB for review and approval.*
SDSU/UCSD Joint Student Review-Reliance Agreements
Review-Reliance agreements are now documented using the SMART Letter of Acknowledgment (LOA). This document may be found at IRB forms.
A brief description of how to initiate review-reliance:
- Determine if the study meets the eligibility to be reviewed under a review-reliance agreement. Only studies that are eligible for expedited review or are determined to be minimal risk at a fully-convened IRB meeting are eligible for review-reliance. SDSU-UCSD JDP Eligibility Facts
- Determine whether SDSU or UCSD is the reviewing IRB. The Reviewing IRB is determined by the primary institutional affiliation of the faculty mentor responsible for oversight of the proposed research.
- Submit the research proposal to the reviewing IRB using the IRB e-submission platform. Include the SMART LOA with the IRB submission as a supporting document.
- The review process if SDSU is the reviewing IRB is the same as the process for SDSU faculty PIs. Questions regarding the process at UCSD can be directed to[email protected].
- At SDSU, once the IRB has reviewed and approved the submission, the SMART LOA will be routed for signature by both SDSU and UCSD. Once signed by both UCSD and SDSU, the research can commence. Note: If UCSD is the reviewing IRB the student PI should contact the UCSD IRB for detailed information regarding UCSD's reliance process.
*Failure to follow the steps outlined above, may result in delayed IRB approval.*
Smart IRB Standard Operating Procedures (SOP)
Standard operating procedures (SOP) for establishing and implementing reliance provide clarity during the review and conduct of research using the SMART IRB Agreement. The SOP contains a search-able index for locating relevant information quickly. It outlines the responsibilities investigators and study teams, of the Reviewing IRB, the Relying IRB, and Points of Contact (POC) for the Reviewing and the Relying IRBs.
Note: The SMART IRB platform is for documenting review-reliance when multiple institutions are engaged in human subjects research. The SMART IRB platform is not the SDSU IRB submission platform. SDSU IRB submissions should be submitted for review via iRIS. See the Guidance page for more information on the process for submitting to proposals to the SDSU IRB for review.