ClinicalTrials.gov
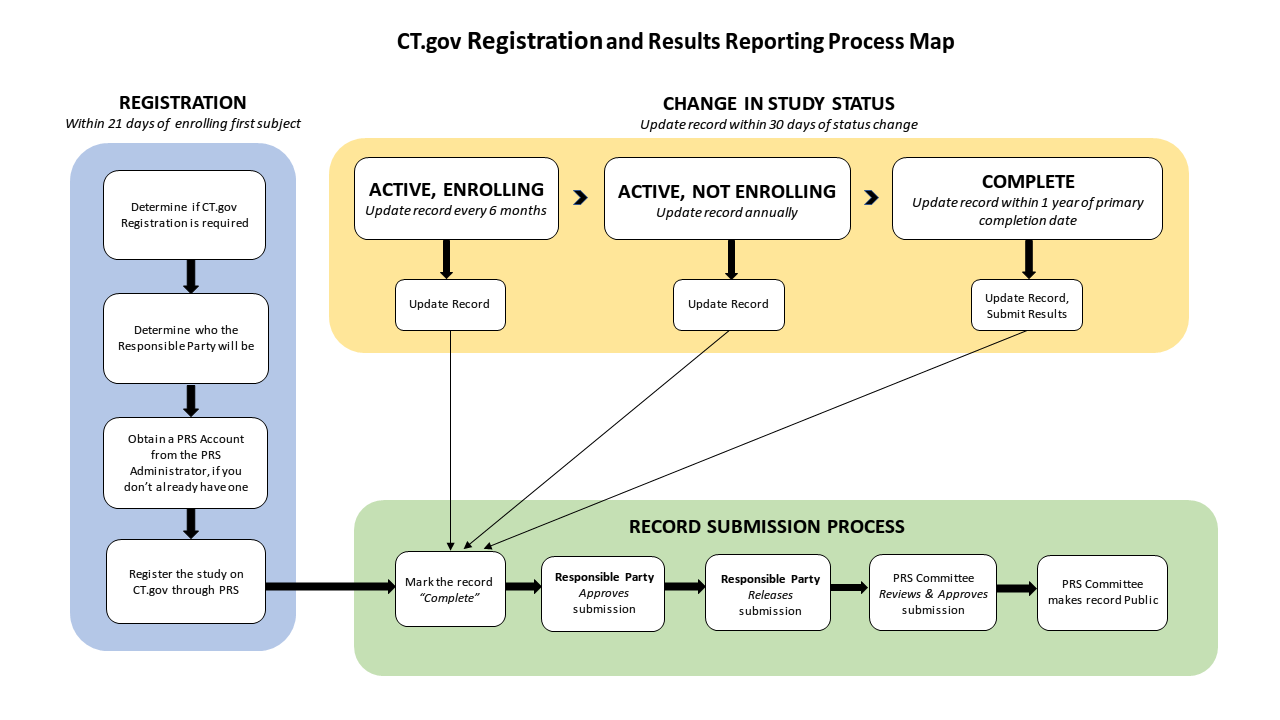
ClinicalTrials.gov is a web-based resource that provides patients/research subjects, their family members, health care professionals, researchers, and the public with easy access to information on federally and privately supported clinical studies on a wide range of diseases and conditions in the United States and across the world. The ClinicalTrials.gov website is maintained by the National Library of Medicine (NLM) at the National Institutes of Health (NIH). Clinical studies are registered on ClinicalTrials.gov via a web-based data entry system called the Protocol Registration System (PRS). Information on ClinicalTrials.gov is provided and updated by the Sponsor or Principal Investigator of the clinical trial. Studies should be submitted to the website (i.e., registered) prior to enrollment of the first subject, and the information on the site is updated throughout the duration of the study. On 11/9/21, NIH issued the Enhanced Checks on Non-Compliance with Clinical Trial Registration Information.
Hot off the PRS: Latest Release and Updates
Data providers use a Web-based data entry system called the Protocol Registration and Results System (PRS) to register clinical studies and to submit results information for registered studies. You must have a PRS account to register study information on ClinicalTrials.gov.
ClinicalTrials.gov establishes one PRS account for an organization (such as SDSU). All investigators from that organization who are conducting studies are typically designated as users of this single PRS account. The organization should designate one or more PRS Administrators to manage the account and to create login privileges for additional users.
Who Should Apply for a PRS Account?
Before applying for a PRS account, you should ensure that you are the appropriate individual to submit clinical study information to ClinicalTrials.gov. To avoid duplicate registration, studies should be registered only by the Responsible Party. To help you determine who is responsible for registering a study and submitting results, see the Elaboration of Definitions of Responsible Party and Applicable Clinical Trial (PDF).
If you are the person responsible for registering a study and submitting results, please find out whether your organization has an existing PRS account before applying for one. To apply for a PRS account, follow the instructions below.
How do I obtain a PRS account?
Fill out this form andEric Morgan will be in contact with you.
1. Determine who is responsible for registering the clinical study and which Protocol Registration and Results System (PRS) account should be used.
2. Learn about submission requirements.
3. Login to PRS.
4. Enter the required and optional data elements.
- For basic help with using PRS, review the Quick Start Guide found in the Help section of the PRS main menu. More detailed instructions are available in the PRS User's Guide.
5. Preview, inspect, and release (submit) the record.
- See the ClinicalTrials.gov Protocol Review Criteria for a description of items that should be addressed before releasing the record to ClinicalTrials.gov.
- Verify in PRS that the Record Status is released. The record will not be processed by ClinicalTrials.gov unless it is released. Only the Responsible Party or a PRS account administrator can release the record.
If you would like step-by-step instructions for entering registration information into the PRS, see the PRS Guided Tutorials. The tutorials include a quick overview guide called Entering a New Registration that briefly summarizes how to use the tutorials to support registering a study.
Ready to report results to ClinicalTrials.gov?
The results data that need to be reported to ClinicalTrials.gov are probably simpler than you think!
Step one is to gather the data that you’ve collected for the following:
- Participant Flow – How many people started versus finished each period of your study and how many dropped out along the way. Most studies only have one period.
- Baseline Characteristics – Demographics data, such as age/sex/ethnicity/race of your participants, and any study-specific baseline measures (for example, “Participant a1c”) that you collected, if any.
- Outcome Measure Data – These are very basic tables of the data that you collected for each of your Outcome Measures. The table rows will be the measurement and the columns will be the arms/groups of participants. You can see sample records with results information here.
- Adverse Events – All Serious AEs, and any AEs that occurred at a frequency over a certain threshold (if any).
Next, follow the PRS Guided Tutorials for Entering Results, which will take you through the process step-by-step.
Frequently Asked Questions
A research study in which one or more human subjects are prospectively assigned to one or more interventions (which may include placebo or other control) to evaluate the effects of those interventions on health-related biomedical or behavioral outcomes. Interventions include drugs, medical devices, procedures, vaccines, as well as noninvasive approaches such as surveys, education, and interviews.
It became a requirement to register all National Institute of Health (NIH) funded clinical trials as of 2007. Even noninvasive approaches may be required to register at clinical trials. See FR 42.CFR part 11.
Even studies not funded by NIH are required by publishers to register high impact journal clinical trials.
Clinical trials testing drugs and devices are required by federal regulations to register their clinical trial on ClinicalTrials.gov; many other clinical trials are now being required by funders and publishers to also register their study.
Federal regulations, 42CFR11, requires all clinical trials testing applicable devices or drugs to register their study on ClinicalTrials.gov prior to beginning recruitment. However, it is becoming more common for all clinical trials, either biomedical or behavioral, to register first on ClinicalTrials.gov.
Before applying for a Protocol Registration and Results System account on ClinicalTrials.gov, you should make sure you're the appropriate person to submit clinical study information. Studies subject to the regulations are registered only by the Responsible Party, although other users can be authorized to add information to the study record either before or after a study is registered.
A clinical trial must be registered on CT.gov if it meets one or more of the following criteria:
- A Food and Drug Administration (FDA) regulated applicable clinical trial (ACT)
- NIH-funded clinical trial
- Plan to publish in an International Committee of Medical Journal Editors (ICMJE) member journal
Each study has one Responsible Party. That responsible party can be either a sponsor, a principal investigator, or a sponsor-investigator.
Sponsor: The entity (for example, corporation or agency) that initiates the study
Principal Investigator: The individual designated as responsible party by the sponsor (see Note)
Sponsor-Investigator: The individual who both initiates and conducts the study
Note: The sponsor may designate a principal investigator as the responsible party if such principal investigator meets all of the following requirements: is responsible for conducting the study; has access to and control over the data from the study; has the right to publish the results of the study; and has the ability to meet all of the requirements for submitting and updating clinical study information.
- Identify studies requiring registry publicly accessible database;
- Create records on ClinicalTrials.gov;
- Confirm accuracy of record content;
- Change ClinicalTrials.gov Record Status following edit completion to trigger release process;
- Ensure ClinicalTrials.gov notification emails are addressed within 15- and 25-day timelines (registration and results records, respectively);
- Maintain records on ClinicalTrials.gov in accordance with all regulatory and policy driven requirements;
- Notify the HRPP Office within 30 days of an expected and 15 days following an unexpected Principal Investigator/Responsible Party personnel change. All changes in Study Official (Principal Investigator), their official title, and/or contact information must be updated in the ClinicalTrials.gov record within 30 days of the change.
HElpful Resources
Contact Eric Morgan for more information