HRPP Guidance
The SDSU HRPP Office has created a new IRB-approved Consent Translation Policy for use in human participant research protocols.
The Consent Translation Policy
- Ensures informed consent be understood by research participants
- Requires translated documents reflect language of study population
- Provides options for translation services
- Defines translation terminology
Steps to IRB Proposal Submission
-
- Take CITI training –CITI Training must be completed by all study members prior to submitting your research proposal to the IRB. Click here for more information on how to sign up.
- Develop your research plan and write your consent form and any other supporting documents
such as recruitment flyers, emails, or scripts and questionnaires or surveys.
- Consent and assent form templates are found here
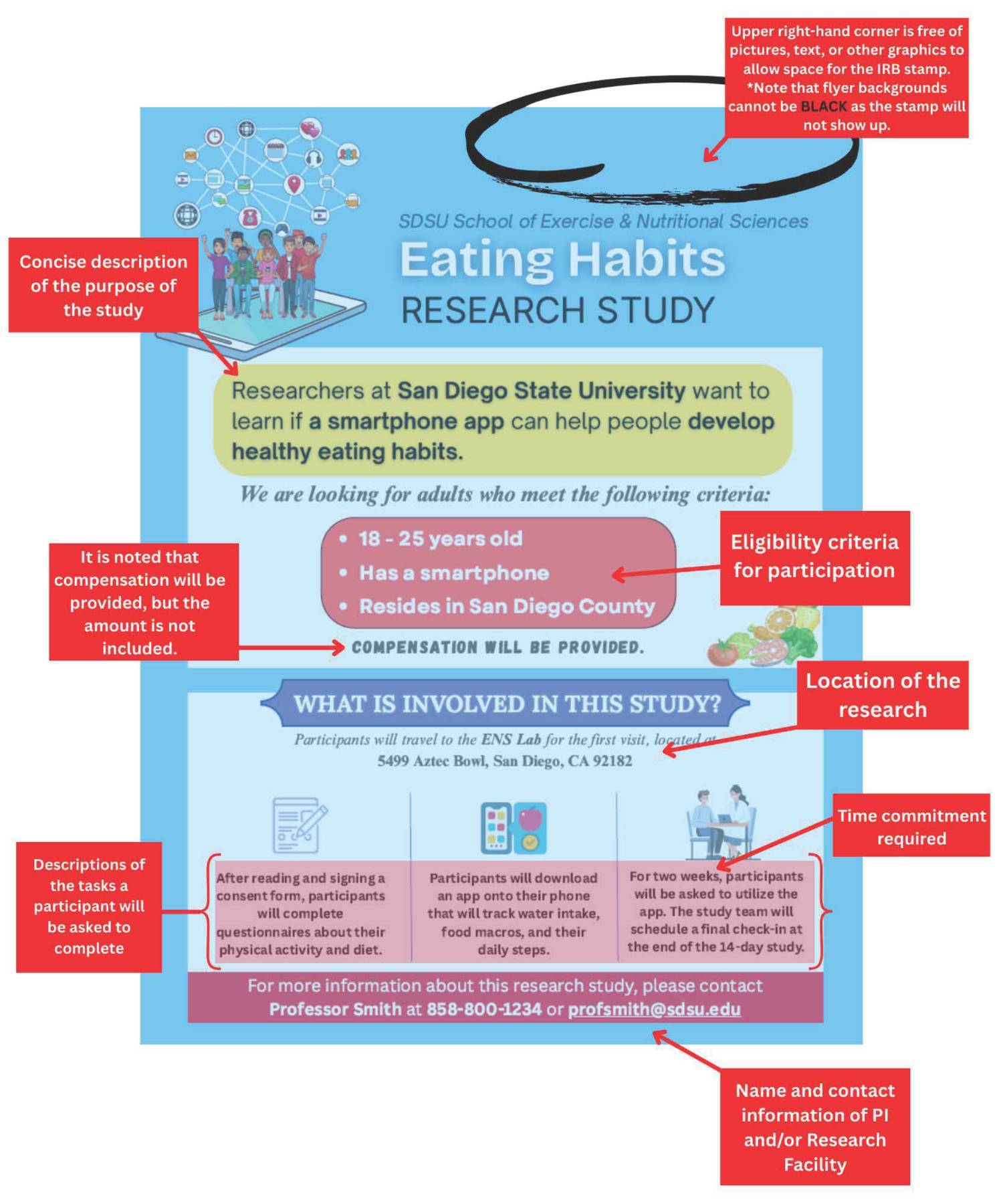
- While the HRPP office does not have templates for research recruitment flyers, emails, or scripts, information on what to include in research recruitment advertisements may be found in the Human Research Program (HRPP) Standards and Practices found at the link below under SDSU IRB Policies and Procedures.
- Submit your research proposal to the IRB via iRIS.
- First time users with an SDSU email do not need to request a new account
Does my Study Require Full Board Review?
Qualtrics Helpful guides
Social identity Demographic guidelines
Due to ongoing changes in campus culture and climate, including in research, the SDSU HRPP Office has put together guidance for researchers of options they can include when collecting data from human research participants.
- Limit demographic questions to those relevant to the research. Before any demographic questions are included in the study materials, first consider why it is important to collect these data. If certain demographic information is not essential to the research question, do not ask participants to provide this information. Researchers should always consider the risk of re-identifying a participant on the basis of specific demographic data (Fernandez et al., 2016). For example, if students from a specific major are recruited to complete a survey, it may be easy to re-identify some students based upon the intersection of their multiple identities.
- Use respectful, non-stigmatizing language. Consult with experts in the field or with members of the community to ensure that the terms being used are as respectful as possible.
- Offer respondents the option to skip a question. For some participants, there may be risk in describing some of their identities. Participants should be given the option to skip demographic questions, if they wish. In an online survey, this can be accomplished by making a response non-mandatory. Another option is to offer a “prefer not to respond” option for all questions. This allows participants control over their disclosure of information and allows participants to take part in a study without disclosing personal information unwillingly.
- Do not use “other” as a possible response. Listing “other” as option communicates to those who do not identify with a listed option that their identity is outside of the norm. This stigmatizes participants whose identities are not listed. Instead, attempt to list inclusive options, rather than list only the most privileged or common identities, and also offer a “prefer to self-identify” option.
- Be comprehensive. When presenting demographic questions to participants that are multiple choice, offer the option to select all that apply. In some cases, it might be feasible to use open-ended questions (Fernandez et al., 2016). This option is inclusive and flexible in that it allows participants to self-identify. However, it may require that researchers spend more time coding these responses, and it may result in fewer answers that can be used in data analysis.
Recruitment Material Policy
Human Research Recruitment Materials PolicyThis document outlines the official policy of the SDSU IRB regarding the creation and use of recruitment materials in human subjects research. Effective as of April 15, 2025, the policy provides clear guidance to SDSU Principal Investigators on the required content, presentation standards, and review procedures for recruitment advertisements such as flyers, social media posts, and other public communications.

Zoom Video Conferencing Best Practices
Information about recording on the cloud and Zoom may be found here.
Note that if you are using the HIPAA compliant version of Zoom this option is disabled.
*For data security purposes and participant privacy, after transcriptions of cloud
recordings, the transcription should be downloaded locally on to your computer, and
then moved to the SDSU Google drive. Finally, recordings saved on the Zoom cloud must
be deleted. Research proposals must be clear and fully describe this when using the
transcription services in Zoom.*
Sensitive Data Storage Best Practices
CSU Record Retention Requirements
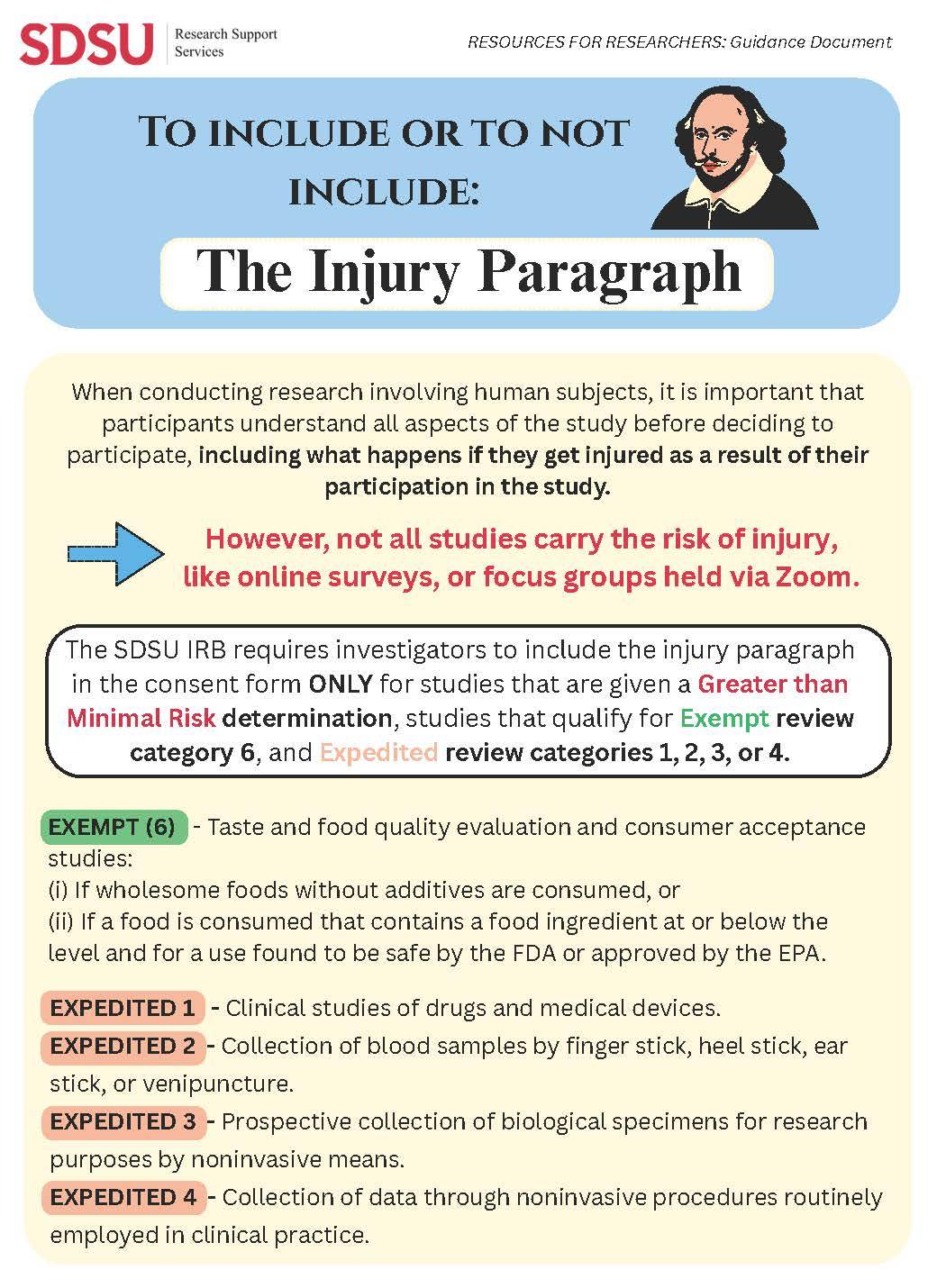
The Injury Paragraph
human Subjects researcher faqs
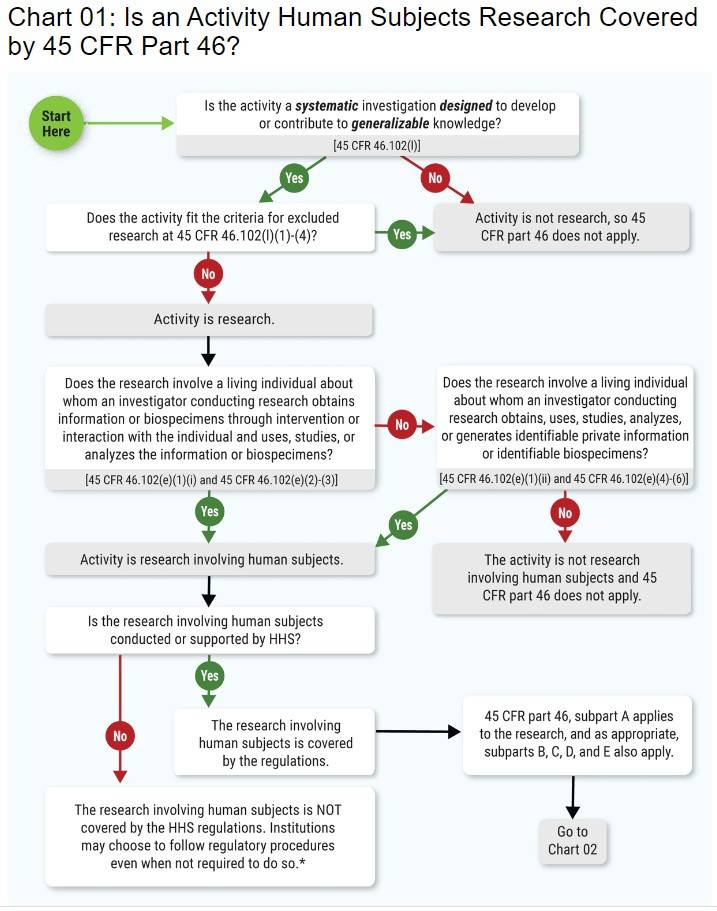
Human subject means a living individual about whom an investigator (whether professional or student) conducting research:
(i) Obtains information or biospecimens through intervention or interaction with the
individual, and uses, studies, or analyzes the information or biospecimens; or
(ii) Obtains, uses, studies, analyzes, or generates identifiable private information
or identifiable biospecimens.
There are three types: full committee review, expedited review, and exempt studies. These protocols are reviewed by the IRB committee at a convened meeting.
Full committee review is required for: Greater than minimal risk studies OR studies that are minimal risk, but do not fit in an expedited review category.
Expedited review studies are reviewed by a small number of IRB reviewers in the HRPP Office. Expedited review is appropriate for studies that according to 45 CFR 46.110 and 21 CFR 56.110: Involve no greater than minimal risk AND fit into one (or more) of the following nine specific expedited review categories.
HHS regulations in 45 CFR 46.104 identify several different categories of minimal risk research as being exempt from federal policy for the protection of human subjects. Federal HIPAA regulations, California state law and SDSU institutional policies further limit exempt research categories.
There are seven expedited categories that may be used for initial IRB review if the research is minimal risk, the research only includes procedures in one or more of the seven categories, and identification of the subjects and/or their responses would reasonably place them at risk of criminal or civil liability or be damaging to the subjects financial standing, employability, insurability, reputation, or be stigmatizing, unless reasonable and appropriate protections will be implemented so that risks related to invasion of privacy and breach of confidentiality are no greater than minimal risk.
The use of categories eight and nine are limited to continuing review.
- Clinical studies of drugs and medical devices only when certain conditions are met
- Collection of blood samples by finger stick, heel stick, ear stick, or venipuncture in certain populations and within certain amounts
- Prospective collection of biological specimens for research purposes by noninvasive means
- Collection of data through noninvasive procedures (not involving general anesthesia or sedation) routinely employed in clinical practice, excluding procedures involving x-rays or microwaves.
- Research involving materials (data, documents, records, or specimens) that have been collected, or will be collected solely for non-research purposes
- Collection of data from voice, video, digital, or image recordings made for research purposes
- Research on individual or group characteristics or behavior or research employing survey, interview, oral history, focus group, program evaluation, human factors evaluation, or quality assurance methodologies
- Continuing review of research previously approved by the convened IRB as follows:where (i) the research is permanently closed to the enrollment of new subjects; (ii) all subjects have completed all research-related interventions; and (iii) the research remains active only for long-term follow-up of subjects; or where no subjects have been enrolled and no additional risks have been identified; or where the remaining research activities are limited to data analysis.
- Continuing review of research, not conducted under an investigational new drug application or investigational device exemption where categories two (2) through eight (8) do not apply but the IRB has determined and documented at a convened meeting that the research involves no greater than minimal risk and no additional risks have been identified.
Your research may be exempt if it meets the criteria in one or more of the categories in the chart below.
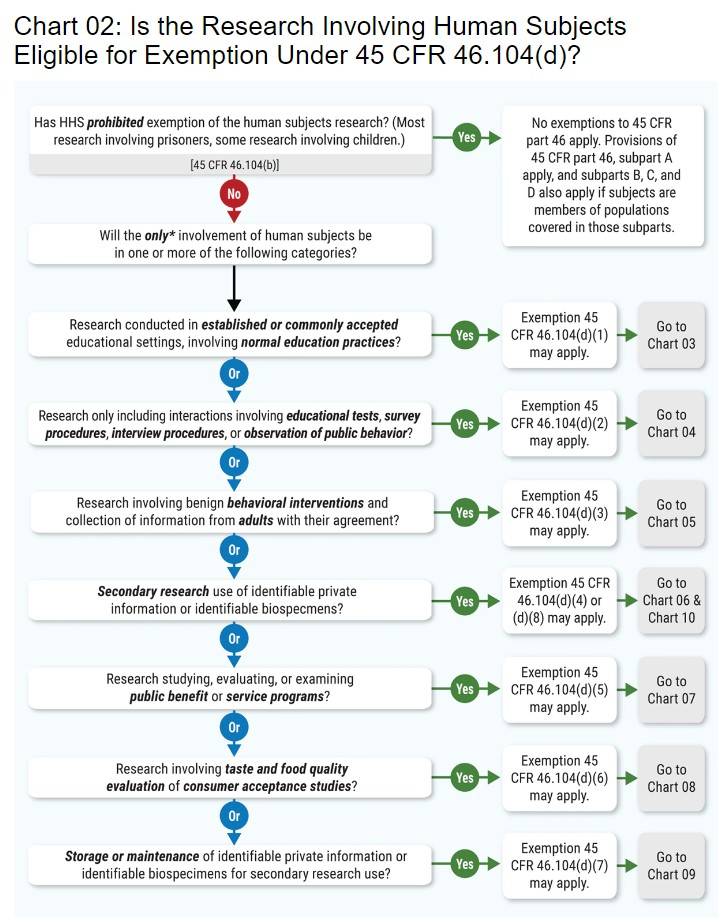
See the OHRP website for the mentioned charts. Contact the SDSUHRPP Office for further guidance if you think your research applies.
Under 45 § 46.102(f), minimal risk means that the probability and magnitude of harm or discomfort anticipated in the research are not greater in and of themselves than those ordinarily encountered in daily life or during the performance of routine physical or psychological examinations or tests.
Greater than Minimal Risk to subjects means that the probability and magnitude of harm or discomfort anticipated in the research risks are more than minimal risk, but not significantly greater. Studies that fall under this category will range in their probability of a moderate-severity event occurring as a result of study participation (and the level of safety monitoring will depend on that probability) but there are adequate surveillance and protections in place to identify adverse events promptly and to minimize harm.
- Data collection for internal departmental, school, or other University administrative purposes. Examples: teaching evaluations, customer service surveys.
- Service surveys issued or completed by University personnel for the intent and purposes of improving services and programs of the University or for developing new services or programs for students, employees, or alumni, if the privacy of the subjects is protected, the confidentiality of individual responses is maintained, and survey participation is voluntary. This would include surveys by professional societies or University consortia. Note: If at a future date, an opportunity arose to contribute previously collected identifiable or coded survey data to a new study producing generalizable knowledge; IRB review may be required before the data could be released to the new project.
- Information-gathering interviews where questions focus on things, products, or policies rather than people or their thoughts regarding themselves. Examples: canvassing librarians about their libraries’ inter-library loan policies or periodical purchases or interviews with company engineers or managers about how a product is made.
- Course-related activities designed specifically for educational or teaching purposes, where data are collected as part of a class exercise or course requirement. If you have any question as to whether your project meets this definition, contact the IRB office prior to beginning the project.
- Biography research involving a living individual that is not generalizable beyond that individual.
- Research involving cadavers, autopsy material or biospecimens from now deceased individuals. Note: Some research in this category, such as genetic studies providing private or medical information about live relatives, may need IRB review. Please contact the IRB for further information.
- Innovative therapies except when they involve "research" as defined by the above criteria.
(An innovative clinical practice is an intervention
designed solely to enhance the well-being of an individual patient or client. The purpose of an innovative clinical practice is to provide diagnosis, preventative treatment, or therapy to individuals, or when the innovative therapy is investigational.) Note: When innovative therapies differ significantly from routine practice it should be viewed and treated as such with appropriate safeguards in place to protect the rights and welfare of the patients. - Quality improvement projects are generally not considered research unless there is a clear intent to contribute to generalizable knowledge and use the data derived from the project to improve or alter the quality of care or the efficiency of an institutional practice. Any individual who is unsure whether a proposed quality improvement project should be classified as research should contact the IRB for guidance. If the data are re-examined or re-analyzed and new information surfaces that would contribute to generalizable knowledge, an application must be submitted to the IRB.
- Case history or Case Study which are published and/or presented at national or regional meetings are not considered research if the case is limited to a description of the clinical features and/or outcome of a three or fewer patients and do not contribute to generalizable knowledge. Note: Investigators should contact the IRB if they are uncertain as to whether they are contributing to generalizable knowledge.
- Publicly available data do not require IRB review. Examples: census data, labor statistics. Note: Investigators should contact the IRB if they are uncertain as to whether the data qualifies as “publicly available.”
- Coded private information or biological specimens that were not collected for the currently proposed projects do not need IRB review if the investigator cannot link the coded data/specimens back to individual subjects. If the data/specimen provider has access to the identity of the subjects (e.g., subjects’ names, addresses, etc.), the investigator must enter into an agreement with the data/specimen provider that states under no circumstances will the identity of the subjects be released to the investigator. Note: Investigators cannot independently make this determination. These projects require verification from the IRB.(http://www.hhs.gov/ohrp/policy/checklists/decisioncharts.html#c1)
- Some examples of Non-Engagement in Research include when an institution’s employees or agents act as consultants on research but at no time obtain, receive, or possess identifiable private information, perform commercial services for the investigators, or inform prospective subjects about the availability of research.
Note: the examples above are not an all-inclusive listing.
In order to grant approval to a research study, the IRB must find and document that the following criteria are met (per 45 CFR 46.116(a)(b)) at the time of initial approval and sustained through continuing review and requests for an amendment:
- Risks to participants are minimized: (i) by using procedures which are consistent with sound research design and which do not unnecessarily expose participants to risk, and (ii) whenever appropriate, by using procedures already being performed on the participants for diagnostic or treatment purposes;
- Risks to participants are reasonable in relation to anticipated benefits, if any, to participants, and the importance of the knowledge that may reasonably be expected to result. In evaluating risks and benefits, the IRB should consider only those risks and benefits that may result from the research (as distinguished from risks and benefits of therapies participants would receive even if not participating in the research). The IRB should not consider possible long-range effects of applying knowledge gained in the research (for example, the possible effects of the research on public policy) as among those research risks that fall within the purview of its responsibility;
- Selection of participants is equitable. In making this assessment the IRB should take into account the purposes of the research and the setting in which the research will be conducted and should be particularly cognizant of the special problems of research involving International Research or Vulnerable Populations, such as children, incarcerated, pregnant participants, cognitively impaired persons, or economically or educationally disadvantaged persons.
- Informed consent will be sought from each prospective participant or the participant’s legally authorized representative, in accordance with, and to the extent required by regulations (or a request to waive or alter the elements of consent must be approved);
- Informed consent will be appropriately documented, in accordance with, and to the extent required by regulations;
- When appropriate, the research plan makes adequate provision for monitoring the data collected to ensure the safety of participants; and
- When appropriate, there are adequate provisions to protect the privacy of participants and to maintain the confidentiality of data (this criterion applies to all studies).
- When some or all of the participants are likely to be vulnerable to coercion or undue influence, such as children, incarcerated, pregnant participants, cognitively impaired persons, refugees, or economically or educationally disadvantaged persons, additional safeguards have been included in the study to protect the rights and welfare of these participants.
No. The federal regulations do not allow for IRB approval of research that has already been conducted. If data was collected for purposes that the IRB determines to be non-research (e.g. program evaluation not initially intended to be used for research), IRB approval can be sought for the data analysis going forward.
It is the responsibility of the Principal Investigator to assess events that occur during the course of a research protocol. Unexpected problems or events whose nature, severity and frequency are not described in the information provided to the IRB or to the subjects must be submitted to the IRB for consideration if they involve risk to the participants or others and are related to either a research intervention or interaction or to the conduct of the study in general. Examples include, but are not limited to: subject experiences, new scientific findings, unexpected complications, missteps in study procedures, or in consent documentation, or breaches of confidentiality. The PI must determine which of the following descriptions apply. The IRB will review reports and make a final determination, indicating agreement or disagreement with the PI’s assessment, and reasons for the determination.
An adverse event is an event that occurs during the course of the research that either causes physical or psychological harm or increases the risk of such harm or results in a loss of privacy or confidentiality to a research participant or to others. The IRB must determine with the help of the PI if such events are anticipated or unanticipated, and also if they are serious and related to the research.
Adverse Events that occur at the University or at an off-campus study site are required to be reported to the IRB on a Reportable Event Report, within the following time frames:
- If Serious and Related to the Research, within 48 hours of discovery of the event.
- For all other events, within 5 days of discovery of the event.