research data sharing
Tools and Guidance for Data Management Plans and Data Sharing

WH Office of science & Tech Policy Public Access Memo
NIH Data Management & Sharing Policy
Get an overview of the 2003 NIH Data Sharing policy and the 2023 NIH Data Management and Sharing policy as well as how they apply to NIH funded research and data. Here you can find out where to get help with data sharing, stay up to date on NIH data sharing policy-related statements, news, and events, and look for training opportunities.
Consider taking this course if you:
- Want to standardize the data you collect so it can be easily shared with and reused by colleagues and other researchers
- Have been awarded a grant that requires the use of common data elements (CDEs)
- Have heard that CDEs can be part of a compliant data management and sharing plan
- Assist researchers with the research data management lifecycle
In this NIH Webinar, you will learn about DMS policy expectations, the applicability of the policy, how to prepare a Data Management and Sharing Plan, and considerations for sharing data responsibly.
In this Webinar, NIH took a deeper dive into the details of the DMS Policy including information such as considerations for privacy protections for sharing human participant data, working with American Indian and Alaska Native communities, how the DMS Policy interacts with the Genomic Data Sharing Policy, and more!
Get Research Assistance from a Subject Librarian
Quick Start
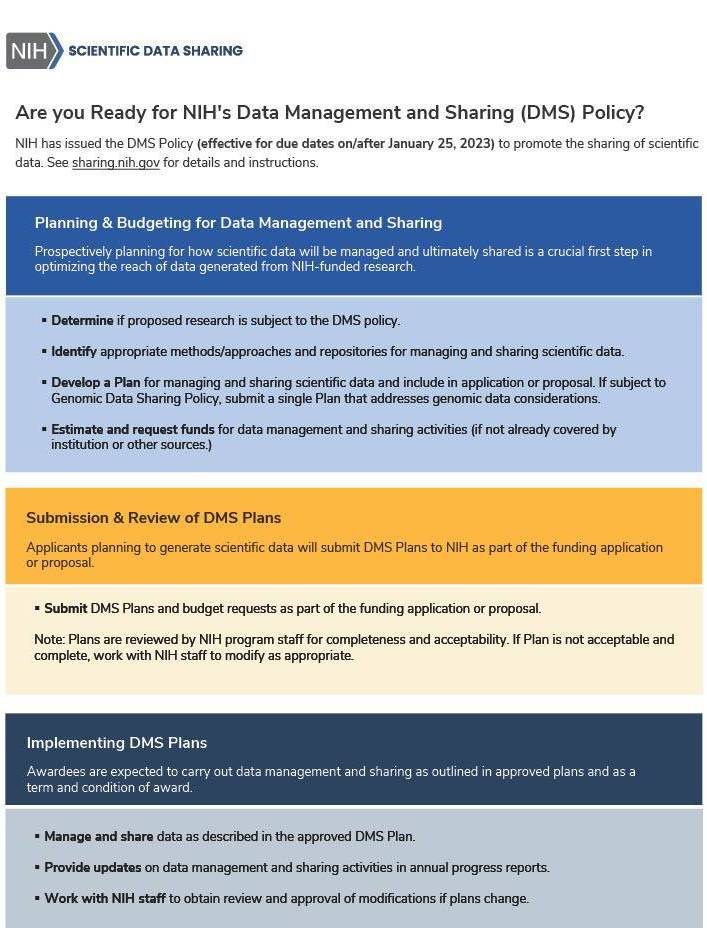
Previously, the NIH only required grants with $500,000 per year or more in direct costs to provide a brief explanation of how and when data resulting from the grant would be shared.
The 2023 policy is entirely new. Beginning in 2023, ALL grant applications or renewals that generate Scientific Data must now include a robust and detailed plan for how you will manage and share data during the entire funded period. This includes information on data storage, access policies/procedures, preservation, metadata standards, distribution approaches, and more. You must provide this information in a data management and sharing plan (DMSP). The DMSP is similar to what other funders call a data management plan (DMP).
The DMSP will be assessed by NIH Program Staff (though peer reviewers will be able to comment on the proposed data management budget). The Institute, Center, or Office (ICO)-approved plan becomes a Term and Condition of the Notice of Award.
- Determine your personal timeline. If you have an active NIH award going up for renewal with receipt date of January 2023, or if you are planning to submit an NIH proposal this year, then developing a DMSP should be a high priority, especially if you are working with external collaborators as it may take time to set up appropriate data procedures/agreements.
- Read through this webpage to familiarize yourself with the changes and with the policy itself (including the supplements)
- Familiarize yourself with the FAIR principles (Wilkinson et. al, 2016). The FAIR (findable, accessible, interoperable, reusable) data principles are the guiding principles the NIH has used in creating the new policy.
- Assess your own project and data management practices relative to the policy, especially around documenting existing practices and developing new ones to address the increased emphasis on data sharing and administrative oversight.
- Review campus data services (e.g., computing, storage, consulting) and assess whether they will meet your needs. Also consider costs you may need to budget for such as labor for data cleaning and documentation.
If you plan to generate scientific data, you must submit a Data Management and Sharing Plan to the funding NIH ICO as part of the Budget Justification section of your application for extramural awards.
Your plan should be two pages or fewer and must include:
- Data Type
- Related Tools, Software and/or Code
- Standards
- Data Preservation, Access, and Associated Timelines
- Access, Distribution, or Reuse Considerations
- Oversight of Data Management and Sharing.
See Supplemental Information to the NIH Policy for Data Management and Sharing: Elements of an NIH Data Management and Sharing Plan for a detailed description of these Elements.
Regardless of funder, these are the five major questions any DMP should answer (adapted from the NSF General Guidelines for data management plans)
- What type of data will be produced?
- How will it be organized and what standards will be used for documentation and metadata?
- What steps will be taken to protect privacy, security, confidentiality, intellectual property or other rights?
- If you allow others to reuse your data, how, where and when will the data be accessed and shared?
- Where will the data be archived and preserved and for how long?
- Learn about the general aspects of DMPs - Understand the kind of information funders usually ask for.
- Check the requirements for your funder - Always check the specific grant solicitation you're applying to for any requirements beyond the standard ones for that funder
- Write the DMP - See exampes for successful DMPs.
- Get Feedback